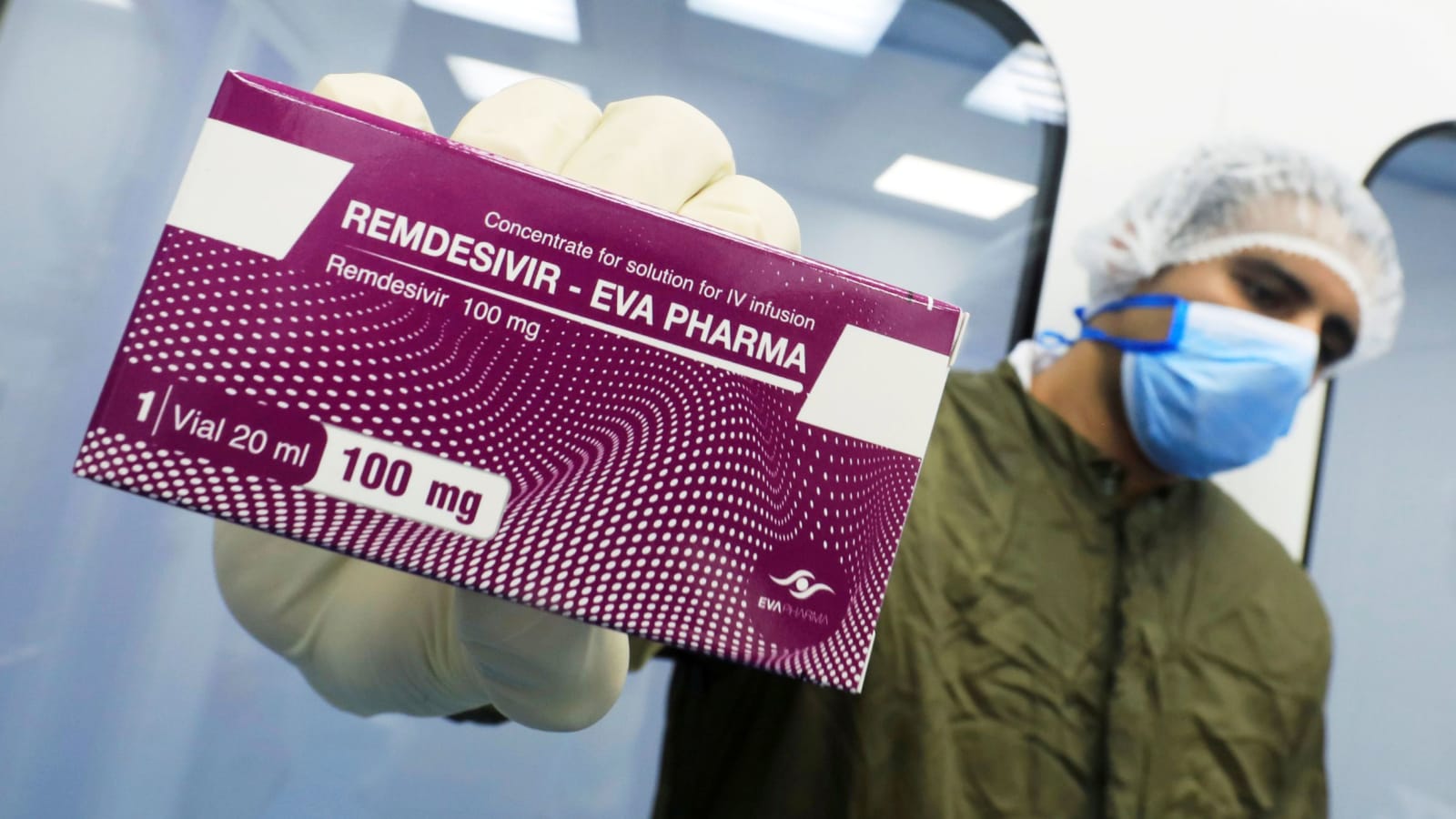
The drug remdesivir has become the first drug to be approved by The US Food and Drug Administration for the treatment of coronavirus infection, it was announced late Thursday evening.
“Today’s approval is supported by data from multiple clinical trials that the agency has rigorously assessed and represents an important scientific milestone in the COVID-19 pandemic,” FDA Commissioner Dr. Stephen Hahn said in a statement.
“As part of the FDA’s Coronavirus Treatment Acceleration Program, the agency will to continue to help move new medical products to patients as soon as possible, while at the same time determining whether they are effective and if their benefits outweigh their risks.”
The drug, better known as Veklury, has been used under emergency use authorization.
“In the United States, Veklury is indicated for adults and pediatric patients (12 years of age and older and weighing at least 40 kg) for the treatment of COVID-19 requiring hospitalization,” Gilead Sciences said in a statement.
“Veklury should only be administered in a hospital or in a healthcare setting capable of providing acute care comparable to inpatient hospital care.”
The drug is given through an IV and should only be administered in a hospital or in a health-care setting capable of providing care comparable with in-patient hospital care.
The news comes following a study sponsored by the World Health Organization found remdesivir did not help patients survive or recover at a faster rate.
The company said it is meeting the real-time demand for the drug in the United States and anticipates meeting global demand this month.