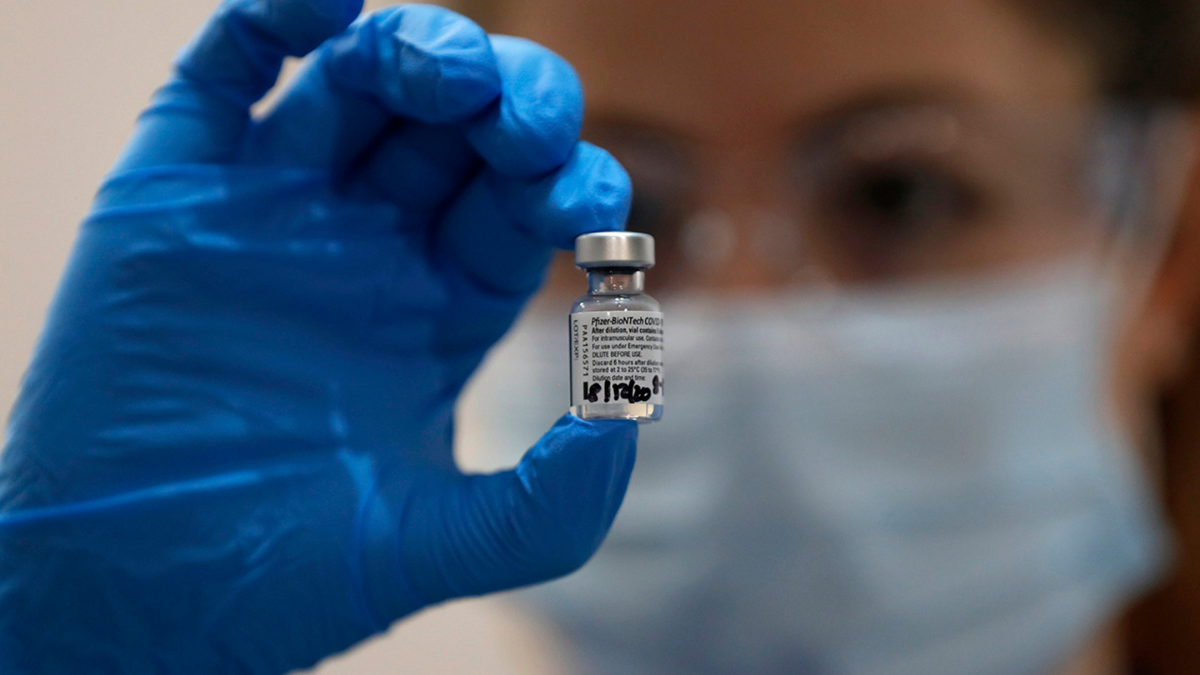
The US Food and Drug Administration has approved the Pfizer and BioNTech coronavirus vaccine for emergency use in the United States, it was announced late Friday night.
The FDA announced the approval came following a recommendation from its Vaccines and Related Biological Products Advisory Committee. The FDA’s approval will now begin a rapid process for the federal government’s distribution of the vaccine to 64 states, territories and major cities across the country.
President Donald Trump tweeted out a video, calling the news “medical miracle”.
The government is currently planning to distribute 2.9 million doses of the vaccine within 24 hours and will follow that up with 2.9 million doses 21 days later for those patients to receive their second shot.
The news of the vaccine getting emergency approval comes as the coronavirus pandemic surging across the country at a higher rate than ever before. Hospitals across the nation are seeing more infected patents now than ever before and the outbreak is expected to set even more grim records.
The director of the Centers for Disease Control and Prevention, Dr. Robert Redfield had a grim warning for the country earlier this week, saying next few months of the pandemic would be “the most difficult in the public health history of this nation.”
The U.S. joins the U.K. and several other countries to try and vaccinate as many people as possible ahead of what is expected to be a very dark winter for the country with the coronavirus spiking in several states.
A vaccine made by Moderna, Inc. is under consideration by the U.S. that could be ready in a week. Meanwhile, Johnson & Johnson is expecting to learn if its vaccine is working in final testing in January.
Europe is also expected to make its own decision on the Pfizer-BioNTech later this month. Friday Sanofi and GSK announce their early tests showed their vaccine didn’t work well enough in older adults.